

In a recent publication Universal Reversible Hydrogen Potential for Electrocatalytic Ammonia Splitting Reactions in Nonaqueous Solvents from Unified pH Measurements. Inorg. Chem. 2025, https://doi.org/10.1021/acs.inorgchem.5c02177, jointly with colleagues from Michigan State University, we have used careful pHabs measurements of dilute NH4+/NH3 buffer solutions in four nonaqueous solvents – acetonitrile (MeCN), Tetrahydrofuran (THF), dimethylformamide (DMF), and propylene carbonate (PC) – to determine the pHabsH2O values aligned to the aqueous pH scale (see the resulting pHabs “ladder” in the graph below). From those measurements (combined with some other experiments) it was possible to determine the reversible hydrogen potential E°H+/H2 in these four solvents relative to the aqueous standard hydrogen electrode (SHE) and, most importantly, ensuring comparability across the different solvents. As an independent method, Open Circuit Potential measurements were carried out in the same solvents titrated with NH4+/NH3 to obtain alternative values for the reversible hydrogen potential in these solvents. The results of the two methods agreed well.
Th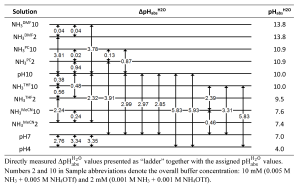 e reversible hydrogen potential values were then used to obtain, for the first time, the overpotential for ammonia oxidation as a function of solvent, with a recently discovered ruthenium catalyst. I.e., it is now for the first time possible to rigorously compare the oxidation process of NH3 to N2 between different solvents!
e reversible hydrogen potential values were then used to obtain, for the first time, the overpotential for ammonia oxidation as a function of solvent, with a recently discovered ruthenium catalyst. I.e., it is now for the first time possible to rigorously compare the oxidation process of NH3 to N2 between different solvents!
This work is a clear demonstration of the usefulness of the unified pH (pHabs) concept in understanding and modelling electrocatalysis processes!
Many thanks, Jaan for performing the extremely difficult pHabs measurements, Agnes for leading this pHabs/electrocatalysis topic in our group and Michigan colleagues for the great collaboration!

Dear Professor ,
I hope this message finds you well. My name is Muhammad Ilyas, and I have recently come across your insightful publication on unified pH (pHabs) measurements in electrocatalysis research, which I found highly impressive and aligns closely with my academic interests.
I have completed my Bachelor of Science in Chemistry with a CGPA of 3.91, and I am very keen to further deepen my expertise in this exciting area. I am writing to inquire whether you might have any funded master’s position(s) available in your research group.
I would be grateful for the opportunity to contribute to and learn from your pioneering work, and I am happy to provide any additional information or documentation you may require.
Thank you very much for your time and consideration. I look forward to your kind response.
Best regards,
Arooba firdos
aroobailyas21@gmail.com