The 2026 edition of the web course (MOOC) Estimation of Measurement Uncertainty in Chemical Analysis will run from March 24 to May 6, 2026. Registration is now open!
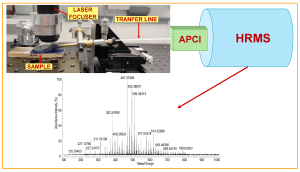
 The full course material (as well as the registration link) is accessible from the web page. The course materials include videos, schemes, calculation files, and numerous self-tests (among them also full-fledged measurement uncertainty calculation exercises) and examples. Almost all areas of analytical chemistry are addressed, ranging from simple volumetric operations and titrations to sophisticated instrumental analysis, such as determining pesticide residues by LC-MS. Efforts are made in the course to address also such uncertainty sources encountered in chemical analysis that are difficult to quantify, e.g. uncertainty due to possible interference effects (incomplete selectivity), analyte losses, etc.
The full course material (as well as the registration link) is accessible from the web page. The course materials include videos, schemes, calculation files, and numerous self-tests (among them also full-fledged measurement uncertainty calculation exercises) and examples. Almost all areas of analytical chemistry are addressed, ranging from simple volumetric operations and titrations to sophisticated instrumental analysis, such as determining pesticide residues by LC-MS. Efforts are made in the course to address also such uncertainty sources encountered in chemical analysis that are difficult to quantify, e.g. uncertainty due to possible interference effects (incomplete selectivity), analyte losses, etc.
In order to pass the course, the registered participants have to take six graded tests and get a higher than 50% score in every graded test. These tests are available to registered participants via the Moodle e-learning platform.
Participants who successfully pass the course will get a certificate from the University of Tartu. A digital certificate of completion is free of charge. A certificate of completion on paper can be requested for a fee of 62 euros.
You are welcome to distribute this message to potentially interested people!