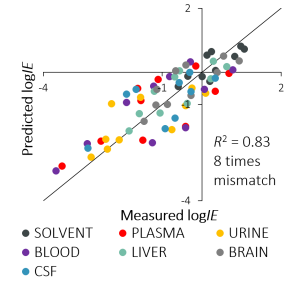In the recent years, we have made significant progress in ionization efficiency studies. In our previous publications, we have shown that our approach can be applied in both electrospray positive and negative mode (we have recently also enabled comparing the logIE values measured in both ESI modes numerically), that our approach is transferable between different instruments with various ESI source geometries from all major mass spectrometric systems vendors, and that it is also transferable between different eluent compositions.
The logIE approach is already applicable in nice clean matrices such as neat solvents. We wanted to see if our approach also works with more difficult matrices, such as bodily fluids and tissues. The results were recently published in Analytica Chimica Acta.
In this study, we took a representative set of 10 compounds, including drugs, e.g., naproxen and lincomycin. As matrices, we used blood, plasma, urine, cerebrospinal fluid, brain and liver tissue homogenates and neat solvent to compare with. We carried out a simple and robust sample pretreatment of protein precipitation. We measured the IEs in a worst-case scenario in flow injection mode without any chromatographic separation.
As with different instrumental setups and solvents, the IEs vary between biological matrices, but the order of the compounds remains roughly the same. These variations between different matrices and variations between a certain biological matrix and neat solvent demonstrate that matrix affects ionization efficiencies and also the prediction models of ionization efficiencies. This, in turn, shows that matrix affects the importance of properties of compounds in the prediction model.
Even though the effects are big and matrix effect is strong we were happy to see that the correlations between IEs measured in the neat solvent and IEs measured in a biological matrix are in good correlation (R2 from 0.7 to 0.9). These good correlations were a promising start to predict IEs in biological matrices similarly to previous predictions in the neat solvent. The most accurate model was obtained for the solvent with a mismatch of 2 times which was also expected since it is the cleanest matrix. But also for liver and brain tissues the mismatch of the model is only 3-fold.
The correlation between predicted and calculated IEs is good with the average mismatch over all biological matrices of 8 times. This means that the accuracy of standard substance free quantitation has been improved by more than an order of magnitude for the set of compounds used in this study.
More details can be found in the paper published in Analytica Chimica Acta. Piia Liigand also gave a talk on the topic in ASMS which can be found here. More papers by our group on the topic of ionization efficiencies can be found here.