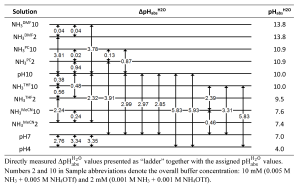
 More than 9000 quality-evaluated pKa values of more than 5000 acids in 7 dipolar aprotic solvents (DMSO, MeCN, DMF, pyridine, acetone, propylene carbonate and THF) have been collected from around 800 original works and are now available as an IUPAC technical report Acid dissociation constants in selected dipolar non-hydrogen-bond-donor solvents. Pure and Applied Chemistry. 2025, https://doi.org/10.1515/pac-2024-0276. The widest possible selection of compound classes is covered (Table below). The results of this large-scale pKa data collection and evaluation work are now available for the scientific community to use in reaction mechanism analysis and modelling, catalyst design, computational method development, etc.
More than 9000 quality-evaluated pKa values of more than 5000 acids in 7 dipolar aprotic solvents (DMSO, MeCN, DMF, pyridine, acetone, propylene carbonate and THF) have been collected from around 800 original works and are now available as an IUPAC technical report Acid dissociation constants in selected dipolar non-hydrogen-bond-donor solvents. Pure and Applied Chemistry. 2025, https://doi.org/10.1515/pac-2024-0276. The widest possible selection of compound classes is covered (Table below). The results of this large-scale pKa data collection and evaluation work are now available for the scientific community to use in reaction mechanism analysis and modelling, catalyst design, computational method development, etc.
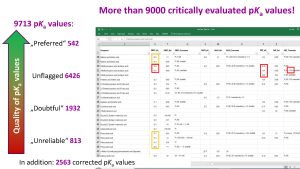
Very importantly, the collected pKa data have been critically evaluated based on predefined quality criteria and depending on situation, kept as they were originally published, flagged as doubtful/unreliable (around 2700 values) or corrected (around 2500 values) (Figure above).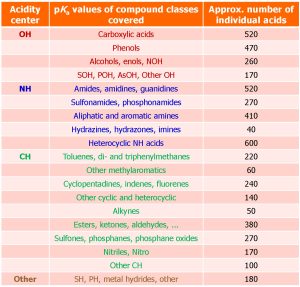
To enable automated processing and data mining, as well as other kinds of cheminformatics, the data are presented as a set of spreadsheets, together with structural codes (SMILES and InChI strings), compound class qualifiers and comments.
The published IUPAC Technical Report contains also comprehensive educational background information on the acid-base processes in non-aqueous media, as well as brief descriptions of the main measurement methods, with focus on the reliability of the data and sources of uncertainty.
The data collection has been deposited in the Zenodo repository and is freely available at https://doi.org/10.5281/zenodo.12608876.
The work has been carried out in the framework of the IUPAC project 2015-020-2-500. It was additionally funded by numerous sources, most importantly the EMPIR programme (project 17FUN09 “UnipHied”, www.uniphied.eu), by the Estonian Research Council grant (PRG690) and by the Estonian Ministry of Education and Research (TK210).